Financial News
Izotropic Files Class II Pre-Submission with FDA & Releases Details on Predicate Devices
 | |||||||||
 |  |  | |||||||
Vancouver, BC – TheNewswire – September 6, 2023 – Izotropic Corporation (“Izotropic” or the “Company”) (CSE:IZO) (OTC:IZOZF) (FSE:1R3), a medical device company commercializing IzoView, a CT (computed tomography) imaging system, that produces images of anatomy for non-invasive tissue characterization with the first application in breast imaging, announced today that it has completed a pre-submission application to the U.S. Food and Drug Administration (FDA) to solidify its plans to initially pursue market clearance for IzoView as a Class II device through a 510(k) pre-market notification submission with the following Indication for Use:
The lzoView CT Imaging System is intended to produce cross-sectional images of anatomy that can be imaged in the 30 cm aperture by computer reconstruction of x-ray transmission data for noninvasive visualization of tissue.
The lzoView CT Imaging System is indicated for use in the non-invasive visualization of breast tissue, as an adjunct tool to mammography, by providing x-ray computer reconstructed images as an aid for qualified healthcare providers.
Upon an anticipated acceptance of the pre-submission application from the FDA, the Company intends to complete the 510(k)-submission using pre-existing data from phantom images obtained from the IzoView system located in its engineering facility in Sacramento, California, later this year, with the objective of obtaining market clearance in the second half of 2024. Receiving this regulatory clearance would enable Izotropic to begin marketing and selling IzoView CT Imaging Systems in the U.S.
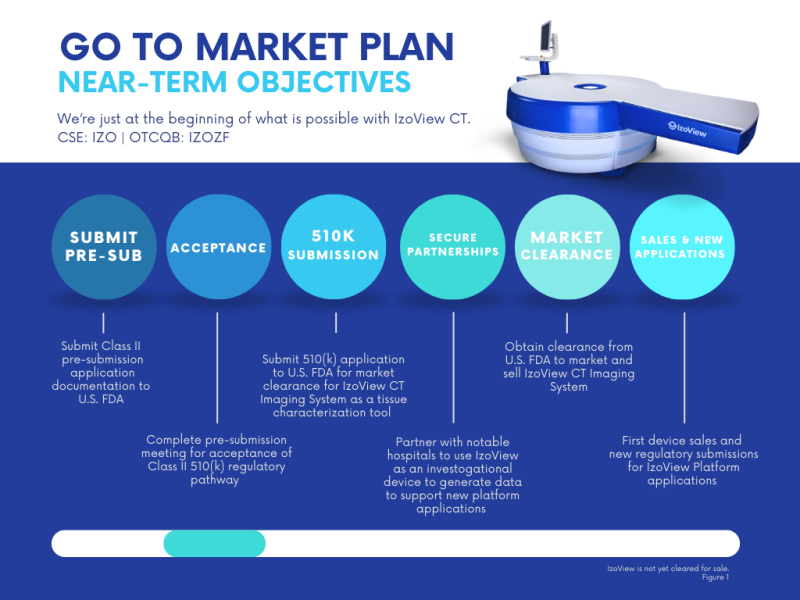
The Company also intends to secure collaborations with notable hospitals to utilize IzoView as an investigational device. Such partnerships are expected to generate clinical data that would support new IzoView products and Indications for Use for new regulatory submissions in the future. See the attached (below) Figure 1: Go To Market Plan.
Explanation of Changes: Class III Pre-Market Approval to Class II 510(k)
In June of 2023, Izotropic announced that it was modifying its FDA market approval pathway and strategy by deferring its plan to undertake a Class III device classification requiring Pre-Market Approval (PMA). The decision to seek regulatory clearance as a Class II device resulted after the completion of an operational plan estimated that costs to market (including a clinical study on human patients) would be three times higher than initially predicted before factoring in operating costs (at least $10+ million in pre-commercial regulatory investment), and the timeline twice as long as initially anticipated at a conservative four years to obtain market approval under the Class III PMA pathway.
Under the Class III pathway, Izotropic was seeking FDA approval for IzoView Breast CT to be used as a stand-alone diagnostic imaging device through a clinical study comparing its capabilities against current standard-of-care breast diagnostic modalities, including diagnostic mammography, tomosynthesis, and breast ultrasound. Under the Class II pathway, Izotropic is seeking FDA clearance for the IzoView CT Imaging System to be indicated for breast tissue characterization, adjunct to mammography, an aid for healthcare providers, with an intended use to produce CT images of anatomy. The IzoView CT Imaging System is fully engineered and is easily retrofitted to accommodate imaging of other body appendages such as hands and feet. The Class II pathway affects both the way Izotropic presents IzoView and the parameters in which IzoView will initially be used by providers in a healthcare setting as a broader investigational imaging device.
Supporting Class II 510(k) Pathway
According to the FDA, a “510(k) requires demonstration of substantial equivalence to another legally U.S. marketed device. Substantial equivalence means that the new device is as safe and effective as the predicate. A device is substantially equivalent if, in comparison to a predicate it: has the same intended use as the predicate; and has the same technological characteristics as the predicate; or has the same intended use as the predicate; and has different technological characteristics and does not raise different questions of safety and effectiveness; and the information submitted to FDA demonstrates that the device is as safe and effective as the legally marketed device. A claim of substantial equivalence does not mean the new and predicate devices needs to be identical. ”
Given these parameters, Izotropic has selected two predicate devices to support it's Class II 510(k) pathway in discussions with the FDA: CurveBeam HiRise and NeuroLogica OmniTom.
The following predicate table, Figure 2: Izotropic Class II Device Submission Predicates, showcases select information, including Intended Use and Indication for Use statements for all three devices. IzoView is comparable, with each system having specific anatomical indications.
|
Device |
Izotropic IzoView |
CurveBeam HiRise |
NeuroLogica OmniTom |
|
Photo |
|
|
|
|
Intended Use & Indication for Use |
The IzoView CT Imaging System is intended to produce cross-sectional images of anatomy that can be imaged in the 30 cm aperture by computer reconstruction of x-ray transmission data for non-invasive visualization of tissue. IzoView is indicated for use in the non-invasive visualization of breast tissue, as adjunct to mammography, as an aid for qualified healthcare professionals. |
The HiRise is intended to be used for 3-D imaging of the upper and lower extremities and pelvis of adult and pediatric patients weighing from 40 to 450 lbs. The device is to be operated in a professional healthcare environment by qualified health care professionals only. |
The NL5000 [OmniTom] system is intended to be used for xray computed tomography applications for anatomy that can be imaged in the 40 cm aperture, primarily head and neck. The CT system is intended to be used for both pediatric and adult imaging and as such has preset dose settings based upon weight and age. The CT images can be obtained either with or without contrast. |
|
510(k) No. |
In Progress |
K203187 |
K171183 |
|
Product Code |
Proposed: JAK (System, X-Ray, Tomography, Computed) |
JAK (System, X-Ray, Tomography, Computed) |
JAK (System, X-Ray, Tomography, Computed) |
|
Principle of Operation |
Cone beam computed tomography x-ray imaging |
Cone beam computed tomography x-ray |
Computed tomography 3D x-ray imaging |
|
Additional Information |
-Seeking FDA Clearance -Comparable gantry, scan axis, aperture bore, radiation shielding (improved for both technologist and general public), and x-ray tubes and additional technical aspects as HiRise and OmniTom - 51,955,021 shares issued |
-FDA Clearance in 2020 -Based in Australia with 170+ device placements -IPO August 2023 ASX: CVB -182,863,995 shares issued |
-16 Slice CT Scanner -FDA Clearance in 2017 -Acquired by Samsung in 2013 for undisclosed terms |
Figure 2: Class II Device Submission Predicates.
Given the similarities to the CurveBeam HiRise and NeuroLogica OmniTom devices that are already cleared for sale in the U.S., Izotropic is proceeding confidently with its plans under the Class II 510(k) regulatory pathway.
ON BEHALF OF THE BOARD
Mr. Robert Thast, CEO
Cell: 604-220-5031
Contact:
Email: info@izocorp.com
Toll Free: 1-833-IZOCORP ext.1
About Izotropic
More information about Izotropic Corporation can be found on its website at izocorp.com and by reviewing its profile on SEDAR at sedar.com.
Forward-Looking Statements
This document may contain statements that are "Forward-Looking Statements," which are based upon the current estimates, assumptions, projections, and expectations of the Company's management, business, and its knowledge of the relevant market and economic environment in which it operates. The Company has tried, where possible, to identify such information and statements by using words such as "anticipate," "believe," "envision," "estimate," "expect," "intend," "may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," "contemplate" and other similar expressions and derivations thereof in connection with any discussion of future events, trends or prospects or future operating or financial performance, although not all forward-looking statements contain these identifying words.
These statements are not guarantees of performance and involve risks, including those related to capital requirements and uncertainties that are difficult to control or predict, and as such, they may cause future results of the Company's activity to differ significantly from the content and implications of such statements. Forward-Looking Statements are pertinent only as of the date on which they are made, and the Company undertakes no obligation to update or revise any Forward-Looking Statements to reflect new information or the occurrence of future events or circumstances unless otherwise required to do so by law. Neither the Company nor its shareholders, officers, and consultants shall be liable for any action and the results of any action taken by any person based on the information contained herein, including, without limitation, the purchase or sale of Company securities. Nothing in this document should be deemed to be medical or other advice of any kind. All images are for illustrative purposes only. IzoView is not yet approved for sale.
____________________________
[1] https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k
Copyright (c) 2023 TheNewswire - All rights reserved.
More News
View More




Recent Quotes
View MoreQuotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.