Financial News
More News
View More
Credo Just Pulled Back—This Might Be the Cleanest Entry Point ↗
Today 13:19 EST
Cash Is King: DigitalBridge Is the Ultimate Defensive Play ↗
Today 12:04 EST
Recent Quotes
View More
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.




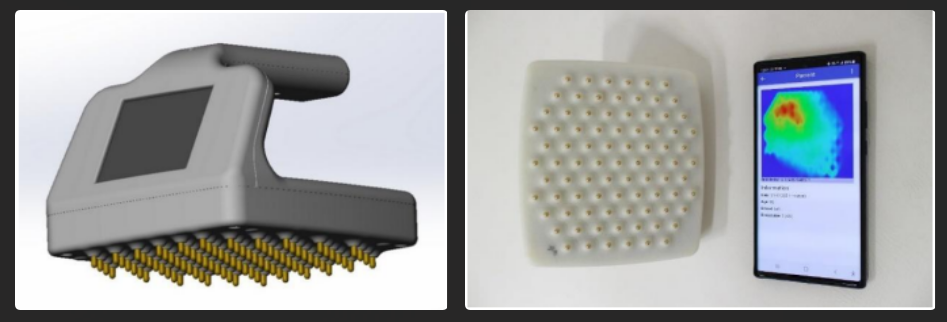
 - The ScanEase OneSense device is a screening tool for diagnosing breast cancer. The device facilitates an examination that is accessible to patients of any age and body type, allowing for self-examination due to its user-friendly design in the privacy of their home. The results help assess the risk of new tissue formations in the breast. The higher the risk factor on the BI-RADS scale, the greater the likelihood of malignant tissue degeneration. In light of this, the OneSense scanning device is recommended for the primary diagnosis of breast tumors in outpatient settings and for at-home self-examinations. The scan results are visible through an app on your smartphone, which will advise whether you should consult with a physician.
- The ScanEase OneSense device is a screening tool for diagnosing breast cancer. The device facilitates an examination that is accessible to patients of any age and body type, allowing for self-examination due to its user-friendly design in the privacy of their home. The results help assess the risk of new tissue formations in the breast. The higher the risk factor on the BI-RADS scale, the greater the likelihood of malignant tissue degeneration. In light of this, the OneSense scanning device is recommended for the primary diagnosis of breast tumors in outpatient settings and for at-home self-examinations. The scan results are visible through an app on your smartphone, which will advise whether you should consult with a physician.



