Financial News
Lantern Pharma Secures FDA Clearance for Planned Phase 1b/2 Trial of LP-184 in Biomarker-Defined, Treatment-Resistant NSCLC Patients with High Unmet Clinical Need
- Drug candidate LP-184 to be evaluated in combination with immune checkpoint inhibitors in biomarker-defined NSCLC patients with KEAP1 and/or STK11 mutations and low PD-L1 expression.
- The FDA has cleared the LP-184 investigational new drug (IND) application amendment for the treatment of a biomarker defined patient population of non-small cell lung cancer (NSCLC).
- The planned phase 1b/2 clinical trial plans to evaluate LP-184 in advanced NSCLC patients with KEAP1 and/or STK11 mutations and low expression of PD-L1 in combination with the immune checkpoint inhibitor therapies, nivolumab and ipilimumab.
- LP-184 is a synthetically lethal, novel small molecule advanced and developed with Lantern’s AI platform, RADR®.
- The median overall survival for newly diagnosed, advanced NSCLC with KEAP1 and/or STK11 mutations treated with checkpoint inhibitors and chemotherapy is estimated to be 15 months and this population presents an annual market opportunity in excess of $2 billion USD.
Lantern Pharma Inc. (Nasdaq: LTRN), an artificial intelligence company developing targeted and transformative cancer therapies using its proprietary AI platform, RADR®, today announced that the U.S. Food and Drug Administration (FDA) has cleared the amendment to its Investigational New Drug (IND) application to initiate a Phase 1b/2 clinical trial of LP-184 in a genomically defined patient population of non-small cell lung cancer (NSCLC) where there is a need to improve patient outcomes.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250512968719/en/
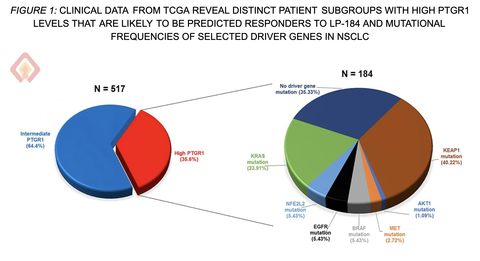
Figure 1: Clinical Data from lung cancer patient groups with High PTGR1 and driver gene mutational status.
“This study represents a potential therapeutic opportunity to improve outcomes for patients diagnosed with advanced non-small cell lung cancer with KEAP1, and STK11 alterations independent of KRAS status. Historically, these co-alterations may lead to worse outcomes for patients, due to resistance to treatments such as single agent immunotherapy, chemotherapy, or chemoimmunotherapy. Research to date suggests that dual immunotherapy is the best approach for this disease type, but many patients still progress on treatment, highlighting an area of unmet need that we can potentially improve on by the addition of LP-184 to this combination,” said Misty Dawn Shields, M.D. Ph.D. — Division of Hematology/Oncology, Thoracic Oncology, Indiana University: Melvin & Bren Simon Comprehensive Cancer Center, and Lead Investigator for the planned Study.
Strategic Trial Design to Address Critical Treatment Gap in Advanced NSCLC
The biomarker focused Phase 1b/2 clinical trial is designed to target high-risk NSCLC subtypes by evaluating LP-184 in combination with the immune checkpoint inhibitors nivolumab and ipilimumab in patients with advanced NSCLC harboring KEAP1 and/or STK11 mutations and low PD-L1 expression—a population with limited response to existing first-line therapies. The study is designed to assess safety, preliminary efficacy, and biomarker correlations, potentially paving a path toward accelerated development in this genetically defined patient segment that is treatment resistant to many existing approved chemo and immunotherapies.1 Lantern Pharma expects to prepare an application for a Fast Track or Accelerated Approval Designation for this patient population based on data from the planned trial and ongoing analysis from existing models.
This unique trial is aimed at addressing a critical unmet clinical need in lung cancer care: the median overall survival in newly diagnosed, advanced NSCLC patients with KEAP1 and/or STK11 mutations treated with chemo-immunotherapy averages 15 months, substantially lower than outcomes in mutation negative populations. For patients that fail earlier lines of therapies the overall survival tends to skew even lower at approximately 6.3 months.2 This represents a market opportunity exceeding $2 billion annually, given the prevalence and poor prognosis for patients with these mutations.
Mechanistic Rationale for LP-184 in NSCLC
LP-184 is a next-generation, synthetically lethal small molecule that induces DNA double-strand breaks upon activation by prostaglandin-reductase 1 (PTGR1). This enzyme is notably overexpressed in KEAP1-mutant tumors. Preclinical studies demonstrate that LP-184's cancer-killing potency directly correlates with PTGR1 expression levels in NSCLC cell lines. The company's proprietary RADR® platform-driven in silico analyses and preclinical studies suggest that LP-184 is particularly effective in DNA damage repair (DDR)-deficient cancers, including NSCLC with KEAP1 and STK11 alterations3. An additional advantage of LP-184 is its selective toxicity mechanism: PTGR1 enzymatically converts LP-184 from a prodrug to its bioactive, cytotoxic form specifically within tumor cells where PTGR1 is elevated, while normal tissues with low PTGR1 expression remain largely unaffected. This selective toxicity has been observed pre-clinically and in Lantern’s AI modeling. This tumor-selective activation creates a significant therapeutic window that may enable robust anti-tumor activity while minimizing systemic toxicities.
Another key competitive advantage of LP-184 is its mechanism, which in preclinical models has been demonstrated to remain effective regardless of TP53 status—a common co-mutation that contributes to resistance against current therapies2. Individual or co-occurring KEAP1, STK11, and TP53 mutations are found in approximately 20-30% of NSCLC patients and define molecular subsets unresponsive to standard immune checkpoint blockade4.
Targeting The Right Patients - Combining Potential Clinical Benefit & Likelihood of Response to LP-184 in a Combination Regimen
Clinical data analysis conducted by Lantern Pharma in 517 lung cancer patients from a major cancer database (TCGA) revealed a clear target population for LP-184. About 35% of these patients had high levels of PTGR1 - the enzyme that has been hypothesized to activate LP-184 inside the cancer cell. (Figure 1)
Among these PTGR1-high lung cancer patients, 40% also had KEAP1 mutations, which are linked to poor responses to current immunotherapies and chemotherapies. KEAP1 mutations actually cause higher PTGR1 expression, essentially making these difficult-to-treat tumors more vulnerable to LP-184 based on preclinical observations.
"This clinical trial and the FDA clearance represents a pivotal milestone in our mission to develop precise, data-driven cancer therapies for patients with limited treatment options," said Panna Sharma, President and CEO of Lantern Pharma. "The STK11 and KEAP1 mutant NSCLC population represents an important market opportunity and, a group of patients desperately awaiting better treatment options. By combining our AI-driven approach with a deep understanding of cancer biology, we've identified LP-184 as a potential breakthrough for these patients. The clearance of this trial advances our precision oncology strategy while demonstrating the power of our RADR® platform to accelerate development timelines and reduce costs, as part of our mission to create value for both patients and shareholders as we work to transform oncology drug development."
About Lantern Pharma
Lantern Pharma (NASDAQ: LTRN) is an AI company transforming the cost, pace, and timeline of oncology drug discovery and development. Our proprietary AI and machine learning (ML) platform, RADR®, leverages approximately 200 billion oncology-focused data points and a library of 200+ advanced ML algorithms to help solve billion-dollar, real-world problems in oncology drug development. By harnessing the power of AI and with input from world-class scientific advisors and collaborators, we have accelerated the development of our growing pipeline of product candidates that span multiple cancer indications, including both solid tumors and blood cancers and an antibody-drug conjugate (ADC) program. On average, our newly developed programs have been advanced from initial AI insights to first-in-human clinical trials in 2–3 years and at approximately $1.0 – $2.5 million per program.
Our lead development programs include a Phase 2 clinical program and multiple Phase 1 clinical trials. We have also established a wholly-owned subsidiary, Starlight Therapeutics, to focus exclusively on the clinical execution of our promising therapies for CNS and brain cancers, many of which have no effective treatment options. Our AI-driven pipeline of innovative product candidates is estimated to have a combined annual market potential of over $15 billion USD and have the potential to provide life-changing therapies to hundreds of thousands of cancer patients across the world.
Forward-Looking Statements:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: future events or our future financial performance; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates and ADC development program; expectations and estimates regarding clinical trial timing and patient enrollment; our research and development efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development process; our intention to leverage artificial intelligence, machine learning and genomic data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate; estimates regarding patient populations, potential markets and potential market sizes; sales estimates for our drug candidates and our plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "model," "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) the risk that we may not be able to secure sufficient future funding when needed and as required to advance and support our existing and planned clinical trials and operations, (ii) the risk that observations in preclinical studies and early or preliminary observations in clinical studies do not ensure that later observations, studies and development will be consistent or successful, (iii) the risk that our research and the research of our collaborators may not be successful, (iv) the risk that we may not be successful in licensing potential candidates or in completing potential partnerships and collaborations, (v) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (vi) the risk that no drug product based on our proprietary RADR® AI platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (vii) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission on March 27, 2025. You may access our Annual Report on Form 10-K for the year ended December 31, 2024 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
1 Skoulidis F, et al. "STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma." Cancer Discov. 2018;8:822–35. 2 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0238358 - “STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world1” 3 Kulkarni A, et al. TCGA analysis of 517 lung adenocarcinoma patients showed elevated PTGR1 in 35% of cases, with 40% of those displaying statistically significant co-occurrence of KEAP1 mutations. Oncotarget. 2021;12(8):785-799.4 Bange E, et al. KEAP1 and TP53 frame genomic, evolutionary, and immunologic subtypes of lung adenocarcinoma with different sensitivity to immunotherapy. Journal of Thorac Oncol. 2021;16:2065-2077. |
View source version on businesswire.com: https://www.businesswire.com/news/home/20250512968719/en/
“This study represents a potential therapeutic opportunity to improve outcomes for patients diagnosed with advanced non-small cell lung cancer with KEAP1, and STK11 alterations independent of KRAS status (...)"
Contacts
Investor Relations
ir@lanternpharma.com
(972) 277-1136
More News
View More




Recent Quotes
View MoreQuotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.



