Financial News
Lantern Pharma Advances Unique ADC (Antibody Drug Conjugate) Program Across Multiple Solid Tumor Cancers
- Lantern, in collaboration with academic research partners, has advanced the development, synthesis, and preclinical proof-of-concept of a novel, highly potent, cryptophycin-based ADC.
- The cryptophycin ADC has shown picomolar potency in a wide range of solid tumors tested in preclinical development and is being further evaluated for clinical potential in six solid tumor indications.
- The company has leveraged RADR®, a proprietary AI platform for oncology drug development, for target selection and molecular payload characterization in ADCs, and a unique, controlled conjugation approach for maximizing drug-to-antibody ratios while controlling for non-specific conjugation.
- Lantern expects to move towards IND development of the ADC program during 2024 with a focus on select solid tumors that are unresponsive or refractory to current therapies.
- ADCs are a promising and emerging class of cancer therapy that are expected to generate over $24 billion in revenue by 2030 and have been the focus of several recent M&A transactions among emerging biotech companies and global pharmaceutical companies.
Lantern Pharma Inc. (NASDAQ: LTRN), a leader in AI-driven cancer drug discovery and development, announced an important milestone in its antibody-drug conjugate (ADC) program. In collaboration with Bielefeld University, Lantern has generated a new class of highly specific and highly potent ADCs with a cryptophycin drug-payload.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240215526567/en/
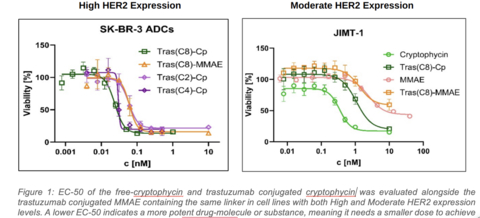
Preclinical results from Lantern's cryptophycin ADC (Cp-ADC) in HER2 expressing cancers in comparison with commercially available MMAE payload antibody-drug conjugates (ADCs). (Graphic: Business Wire)
This novel approach utilizes cysteine-engineered antibodies which allows for the development of uniform and homogenous ADCs with precise control of the drug to antibody ratio (DAR). The drug-payload, cryptophycin, has the potential to improve upon existing ADCs used in the clinical setting by: 1) improving the anti-tumor potency of the ADC molecule, and 2) overcoming drug resistance tumors can frequently develop to existing drug-payloads such as MMAE (Monomethyl auristatin E). The cryptophycin drug-payload and cryptophycin-ADC (CpADC) averaged an 80% cancer cell kill rate across the tested cancer cell lines and significantly outperformed MMAE.
In a broad range of preclinical studies, the cryptophycin-ADC (Cp-ADC) demonstrated promising picomolar level potency and anti-tumor activity in a wide range of solid tumors, including six cancer indications that are being further evaluated. These six indications include: breast, bladder, colorectal, gastric, pancreatic and ovarian cancer. Initial results (Figure 1) have also shown that in high Her2 expressing tumors, Cp-ADC with a DAR of 8 (Tras(C8)-Cp) and DAR of 4 (Tras(C4)-Cp) is more potent than an MMAE ADC with a DAR of 8. MMAE payloads are used in several commercially available anti-cancer ADCs, including Adcetris®, Polivy® and Enhertu®. Additionally, the Cp-ADC with a lower DAR (Tras(C2)-Cp) provides an equivalent tumor kill-rate to that of an MMAE-ADC with a DAR of 8. In a moderate Her2 expressing cell line, the Cp-ADC with a DAR of 8 (Tras(C8)-Cp) was about 10 times more potent than a DAR 8 MMAE-ADC.
Figure 1: EC-50 of the free cryptophycin and trastuzumab conjugated cryptophycin was evaluated alongside the trastuzumab conjugated MMAE (with the same linker) in cell lines with both High and Moderate HER2 expression levels. A lower EC-50 indicates a more potent drug, molecule or substance, meaning it needs a smaller dose to achieve the same effect.
The newly developed Cp-ADC showed highly efficient anti-tumor activity in all six cancer cell lines with EC-50 values in the picomolar to single-digit nanomolar range. Additional studies are now being developed to further validate and expand these findings and to obtain a better understanding of the genomic and biomarker correlates of payload efficacy across these tumors.
Kishor Bhatia, Ph.D., Lantern’s Chief Scientific Officer commented, "Our strategic, data-driven approach of utilizing cryptophycin as a highly potent and novel payload alongside the prioritization of biologically novel and relevant targets with scalable and efficient drug conjugate formats will help expand the repertoire and diversity of ADC opportunities."
Lantern is also utilizing its AI platform, RADR® to further refine and understand other cancer targets, with a focus on prioritizing targets that are expressed across multiple tumor types or subtypes and have few or no therapeutic ADC options. Given the promise of cryptophycin as a payload, Lantern is also focused on the development and testing of two other cryptophycin-ADC molecules. For these selected targets, Lantern is in advanced discussions with potential partners and collaborators with the goal of generating proof-of-concept data for these additional ADCs and potentially other novel drug conjugate formats. Lantern expects to provide additional details on these studies and collaborations in the coming quarters. These efforts aim to improve ADC development for specific patient populations and potentially guide more effective future clinical treatments with less cost and greater efficiency than historical ADC drug development.
About Lantern Pharma:
Lantern Pharma (NASDAQ: LTRN) is an AI company transforming the cost, pace, and timeline of oncology drug discovery and development. Our proprietary AI and machine learning (ML) RADR® platform leverages over 60 billion oncology-focused data points and a library of 200+ advanced ML algorithms to help solve billion-dollar, real-world problems in oncology drug development. By harnessing the power of AI and with input from world-class scientific advisors and collaborators, we have accelerated the development of our growing pipeline of therapies including eleven cancer indications and an antibody-drug conjugate (ADC) program. On average, our newly developed drug programs have been advanced from initial AI insights to first-in-human clinical trials in approximately 2-3 years and at approximately $1.0-2.0 million per program.
Please find more information at:
Website: www.lanternpharma.com
LinkedIn: https://www.linkedin.com/company/lanternpharma/
X: @lanternpharma
Monthly Newsletter: Sign-up here
Forward-looking Statements:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: future events or our future financial performance; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates and ADC development program; expectations and estimates regarding clinical trial timing and patient enrollment; our research and development efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development process; our intention to leverage artificial intelligence, machine learning and biomarker data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate; estimates regarding patient populations, potential markets and potential market sizes; sales estimates for our drug candidates and our plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "model," "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) the risk that our research and the research of our collaborators may not be successful, (ii) the risk that promising observations in preclinical studies do not ensure that later studies and development will be successful, (iii) the risk that we may not be successful in licensing potential ADC candidates or in completing potential partnerships and collaborations, (iv) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (v) the risk that no drug product based on our proprietary RADR® AI platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (vi) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the Securities and Exchange Commission on March 20, 2023. You may access our Annual Report on Form 10-K for the year ended December 31, 2022 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240215526567/en/
Contacts
Investor Relations
ir@lanternpharma.com
More News
View More




Recent Quotes
View MoreQuotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.



