Financial News
GRAIL and University of Oxford to Present Results From First Prospective Study of Multi-Cancer Early Detection in a Symptomatic Patient Population at 2023 ASCO Annual Meeting
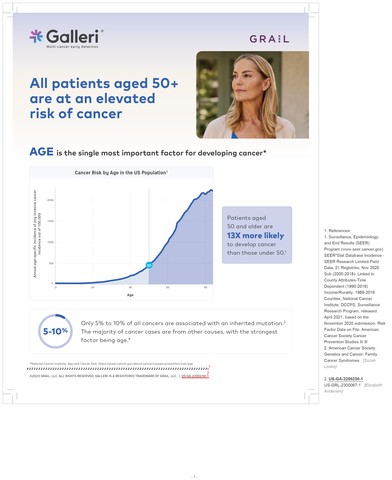
SYMPLIFY Results Demonstrate Strong Performance of GRAIL’s Multi-Cancer Early Detection Methylation-Based Platform in Individuals Presenting With Non-Specific Signs and Symptoms
Results in Press for Publication in The Lancet Oncology
GRAIL, LLC, a healthcare company whose mission is to detect cancer early when it can be cured, and the University of Oxford today announced results from the prospective SYMPLIFY study will be presented during an oral session on Saturday, June 3, at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago. SYMPLIFY is the first large-scale evaluation of a multi-cancer early detection (MCED) test in individuals who presented to primary care and were referred for diagnostic follow-up for suspicion of cancer. The analysis showed strong performance of GRAIL’s MCED methylation-based platform in the symptomatic population of more than 6,000 patients and demonstrated the feasibility of using an MCED test to assist clinicians with decisions around the route of referral from primary care. These results are in press for publication in The Lancet Oncology.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230601006068/en/
(Graphic: Business Wire)
“Earlier cancer detection and subsequent intervention has the potential to greatly improve patient outcomes. Most patients diagnosed with cancer first see a primary care physician for the investigation of symptoms suggestive of cancer, like weight loss, anemia, or abdominal pain, which can be complex as there are multiple potential causes. New tools that can both expedite cancer diagnosis and potentially avoid invasive and costly investigations are needed to more accurately triage patients who present with non-specific cancer symptoms,” said Brian D. Nicholson, MRCGP, DPhil, Associate Professor at the Nuffield Department of Primary Care Health Sciences, University of Oxford, United Kingdom and co-lead investigator of the study. “The high overall specificity, positive predictive value, and accuracy of the cancer signal detected and cancer signal origin prediction that was reported across cancer types in the SYMPLIFY study indicate that a positive MCED test could be used to confirm that symptomatic patients should be evaluated for cancer before pursuing other diagnoses.”
The SYMPLIFY study enrolled 6,238 patients, aged 18 and older, in England and Wales who were referred for urgent imaging, endoscopy or other diagnostic modalities to investigate symptoms suspicious for possible gynecological, lung, lower GI or upper GI cancer, or who had presented with non-specific symptoms. Participants provided a blood sample, from which cell-free DNA was isolated and stored until GRAIL’s MCED test was performed in batches, blinded to clinical outcome. GRAIL’s MCED test’s predictions (cancer signal detected, and if so, cancer signal origin [CSO]) were compared with the diagnosis obtained by standard of care to assess the test’s performance. The most commonly reported symptoms leading to referral were unexpected weight loss (24.1%), change in bowel habit (22.0%), post-menopausal bleeding (16.0%), rectal bleeding (15.7%), abdominal pain (14.5%), pain (10.6%), difficulty swallowing (8.8%) and anemia (7.1%).
Within the study, 368 (6.7%) of the 5,461 evaluable patients were diagnosed with cancer through standard of care. The most common cancer diagnoses were colorectal (37.2%), lung (22.0%), uterine (8.2%), oesophago-gastric (6.0%) and ovarian (3.8%).
GRAIL’s MCED test detected a cancer signal in 323 people, 244 in whom cancer was diagnosed, resulting in a positive predictive value (PPV) of 75.5%, negative predictive value (NPV) of 97.6%, and specificity of 98.4%. The overall sensitivity of the MCED test was 66.3%, ranging from 24.2% in stage I cancers to 95.3% in stage IV, and increased with age and later cancer stage. The overall accuracy of the top CSO prediction after a positive MCED test was 85.2%. The mean age of patients in the study was 62.1 years old.
“GRAIL’s earlier PATHFINDER study previously demonstrated that adding GRAIL’s MCED testing to standard of care screening more than doubled the number of cancers detected compared with standard screening alone in adults with no symptoms or suspicion of cancer. Now, the SYMPLIFY data confirm the potential benefit of methylation-based MCED blood tests as a diagnostic aid for use in the symptomatic patient population,” said Sir Harpal Kumar, President of GRAIL Europe. “These exciting results will inform our development of an optimized classifier for use in symptomatic patients with a suspicion of cancer.”
The University of Oxford sponsored the SYMPLIFY study and was responsible for data collection, analysis and interpretation. The study was funded by GRAIL with support from National Health Service (NHS) England, NHS Wales, the National Institute for Health and Care Research (NIHR) and NIHR Oxford Biomedical Research Centre.
“This is a fantastic example of how academia and industry can work together for patient benefit, recruiting over 6,000 patients to SYMPLIFY in under six months and within less than a year of launching the project,” said Professor Helen McShane, Director of the NIHR Oxford Biomedical Research Centre. “We are committed to diagnosing cancers earlier, when they can be cured, and this study is an important step on that journey. SYMPLIFY also shows that we can run trials at scale using digital systems to deliver research quickly and cost effectively, with the help of the NIHR’s Clinical Research Network.”
About Oxford NIHR Biomedical Research Centre
The National Institute for Health and Care Research (NIHR) Oxford Biomedical Research Centre (BRC) is based at the Oxford University Hospitals NHS Foundation Trust and run in partnership with the University of Oxford.
The mission of the NIHR is to improve the health and wealth of the nation through research. We do this by:
- funding high quality, timely research that benefits the NHS, public health and social care;
- investing in world-class expertise, facilities and a skilled delivery workforce to translate discoveries into improved treatments and services;
- partnering with patients, service users, carers and communities, improving the relevance, quality and impact of our research;
- attracting, training and supporting the best researchers to tackle complex health and social care challenges;
- collaborating with other public funders, charities and industry to help shape a cohesive and globally competitive research system;
- funding applied global health research and training to meet the needs of the poorest people in low- and middle-income countries.
NIHR is funded by the Department of Health and Social Care. Its work in low- and middle-income countries is principally funded through UK Aid from the UK government.
About GRAIL
GRAIL is a healthcare company whose mission is to detect cancer early, when it can be cured. GRAIL is focused on alleviating the global burden of cancer by using the power of next-generation sequencing, population-scale clinical studies, and state-of-the-art machine learning, software, and automation to detect and identify multiple deadly cancer types in earlier stages. GRAIL’s targeted methylation-based platform can support the continuum of care for screening and precision oncology, including multi-cancer early detection in symptomatic patients, risk stratification, minimal residual disease detection, biomarker subtyping, treatment and recurrence monitoring. GRAIL is headquartered in Menlo Park, CA with locations in Washington, D.C., North Carolina, and the United Kingdom. GRAIL, LLC, is a subsidiary of Illumina, Inc. (NASDAQ: ILMN) currently held separate from Illumina Inc. under the terms of the Interim Measures Order of the European Commission.
For more information, visit grail.com.
Laboratory/Test Information
GRAIL’s clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. GRAIL’s clinical laboratory is regulated under CLIA to perform high-complexity testing.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230601006068/en/
Contacts
For GRAIL
Corporate Communications
Kristen Davis
Trish Rowland
pr@grail.com
UK Media
Julie Kangisser
Julie.kangisser@claremont.org.uk
For Oxford NIHR BRC
Susannah Cronin
Roy Probert
susannah.cronin@medsci.ox.ac.uk
More News
View More




Recent Quotes
View More
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.



