Financial News
Greenwich LifeSciences Publishes Additional Positive Safety Data from GP2 Phase IIb Trial at ASCO 2021, Confirming that GP2 Treatment to Prevent Metastatic Breast Cancer Recurrence is Well Tolerated
- Poster published at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting shows the final 5 year safety data from the Phase IIb breast cancer clinical trial.
- Final safety conclusions are that GP2 immunotherapy is well tolerated and that no serious adverse events related to GP2 immunotherapy were reported over the full 5 year follow-up period.
- Final 5 Year Data Set of GP2 Phase IIb Trial is Now Complete: Figure 1 of the poster shows a time series of the GP2 immunotherapy injections, adverse events (AE), immune response, and 100% disease-free survival (0% recurrence rate) in HER2 positive breast cancer patients over median 5 years. This time series highlights that the 10 GP2 immunotherapy injections over the first 2.5 years (as depicted by the 10 arrows) created a potent immune response that peaked at 6 months. The immune response includes injection site and systemic reactions (types of adverse events) that also peaked at 6 months. These adverse events are a positive sign that the immune system responded to GP2 immunotherapy and prevented metastatic breast cancer recurrence. Adverse events were temporally associated with GP2 injections and declined after GP2 injections ended.
Greenwich LifeSciences, Inc. (Nasdaq: GLSI) (the “Company”), a clinical-stage biopharmaceutical company focused on the development of GP2, an immunotherapy to prevent breast cancer recurrences in patients who have previously undergone surgery, presented an abstract and poster of the final 5 year GP2 Phase IIb clinical trial safety data at the 2021 ASCO Annual Meeting.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210607005374/en/
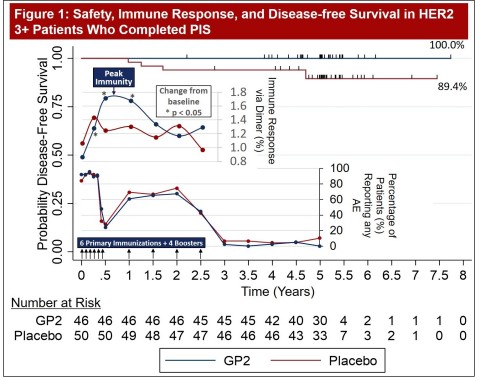
Figure 1 of Poster Presentation 542 from 2021 ASCO Annual Meeting Showing GP2 Immunotherapy 5 Year Time Series of Dosing, Safety, Immune Response, and Disease Free Survival from Phase IIb Clinical Trial (Graphic: Business Wire)
The abstract can be viewed at the bottom of this press release, and the full poster, Figures 1-2, Tables 1-2, and an audio track of the poster by VP of Clinical & Regulatory Affairs Jaye Thompson can be accessed and downloaded at: https://greenwichlifesciences.com/clinical-trials/.
It has been previously reported that the completion of the GP2 immunotherapy (GP2+GM-CSF) Primary Immunization Series (PIS) reduced recurrence rates to 0% over a 5 year follow-up period in HER2 3+ patients who had received a standard course of trastuzumab after surgery. The abstract and poster present the final safety data over the 5 year follow-up period, assessing the safety of GP2 immunotherapy and its relationship to previously presented peak immunity and recurrence rate data.
Summary of the Final 5 Year Safety Data:
- GP2 immunotherapy is well-tolerated and no safety signal for GP2 was identified. Additionally, no serious adverse events related to GP2 immunotherapy were reported over the full 5 year treatment and follow-up periods.
- The majority of patients experienced mild or moderate injection site reactions, which accounted for approximately 70% of reported adverse events.
- The incidence of adverse events was similar across HER2 3+ and HER2 1-2+ breast cancer patients, consistent with the previously reported findings that the immune response was similar across both patient populations, suggesting that GP2 immunotherapy could be a potential treatment in HER2 1-2+ patients or in other HER2 expressing cancers.
Snehal Patel, CEO of Greenwich LifeSciences, commented, “The final 5 year analysis of the safety data in the Phase IIb trial is now complete and represents an important milestone for the Company. With the recurrence rate or disease free survival (SABCS 2020), immune response (AACR 2021), and now safety data (ASCO 2021), the final design of the planned Phase III trial can be completed. This final combined data set encourages us to utilize the same treatment strategy in the planned Phase III trial to conservatively reproduce these promising results that showed that GP2 immunotherapy may prevent metastatic breast cancer recurrence. In addition, this final data set can now be presented to investors and strategic parties interested in partnering with the Company to co-develop and license GP2.”
Excerpts from the ASCO Poster 542:
Title: Final five year median follow-up safety data from a prospective, randomized, placebo-controlled, single-blinded, multicenter, phase IIb study evaluating the use of HER2/neu peptide GP2 + GM-CSF vs. GM-CSF alone after adjuvant trastuzumab in HER2 positive women with operable breast cancer
Safety data was analyzed to assess injection site reactions and systemic adverse events (AEs) of each treatment arm. Most patients completed the planned PIS: 81 (91.0%) GP2+GM-CSF and 86 (94.5%) GM-CSF only. In addition, 77 GP2+GM-CSF and 80 GM-CSF only patients received all 4 booster injections. The most common injection site reactions were erythema, induration and pruritis, and they occurred with similar frequency in the two treatment arms. Injection site reactions were reported by almost all patients over the course of vaccinations. Occurring in a smaller percentage of patients, the most common systemic adverse events were fatigue, headache, and myalgia/arthralgia, again with similar incidence by treatment arm. The majority of all events reported were of grade 1, mild severity. Five GP2+GM-CSF patients reported 6 events considered definitely, possibly or probably related to study medication, which were grade 3 or 4: induration (2), urticaria, rash, pruritis, and arthralgia. Urticaria, allergic reaction and hypersensitivity reaction were considered possibly related events of grade 3 or 4 in GM-CSF only patients. No serious adverse events considered related to study medication were reported over the full 5 year treatment and follow-up periods.
Figure 2 of the poster shows the maximal severity grade for any adverse event, systemic and injection site reaction, for each patient. There was no difference between the two treatment arms. The majority of events were of grade 1, mild severity. Two patients reported grade 4 adverse events deemed unrelated to GP2 immunotherapy. One GP2+GM-CSF patient experienced grade 4 hypoglycemia and recovered. A GM-CSF only patient was diagnosed with renal cell carcinoma, a second primary diagnosis, which was classified as grade 4.
Tables 1 & 2 of the poster show the incidence of adverse events by HER2 status. The first occurrence of frequently reported adverse events are tabulated in Table 1. The most common adverse event was injection site reaction. Almost every patient, in both the GP2+GM-CSF and GM-CSF only arms, reported injection site reactions. The most frequent injection site reactions were erythema, pruritus and induration, as presented in Table 2. The incidence of adverse events were similar across HER2 3+ and HER2 1-2+ patients, which is consistent with the previously reported findings that immune response was similar across HER2 3+ and HER2 1-2+ patients.
ASCO Abstract 542:
Title: Final five-year median follow-up safety data from a prospective, randomized, placebo-controlled, single-blinded, multicenter, phase IIb study evaluating the use of HER2/neu peptide GP2 + GM-CSF vs. GM-CSF alone after adjuvant trastuzumab in HER2 positive women with operable breast cancer.
Snehal Patel, David McWilliams, Christine T Fischette, Jaye Thompson, Mira Patel, and F Joseph Daugherty.
Greenwich LifeSciences, Stafford, TX
Background: The final analysis of the GP2 prospective, randomized, placebo-controlled, single-blinded, multicenter Phase IIb trial investigating GP2+GM-CSF administered in the adjuvant setting to node-positive and high-risk node-negative breast cancer patients with tumors expressing any degree of HER2 (immuno-histochemistry [IHC] 1-3+) (NCT00524277) is now complete with 5 year follow-up. The trial enrolled HLA-A02 patients randomized to receive GP2+GM-CSF versus GM-CSF alone. It was previously reported that completion of the GP2+GM-CSF Primary Immunization Series (PIS) reduced recurrence rates to 0% over a 5 year follow-up period in HER2 3+ patients, who received a standard course of trastuzumab after surgery.
Methods: Each enrolled and consented patient was randomly scheduled to receive a total of 6 GP2+GM-CSF (500 mcg GP2: 125 mcg GM-CSF) or GM-CSF only intradermal injections every 3-4 weeks as part of the PIS for the first 6 months and 4 GP2+GM-CSF or GM-CSF only booster intradermal injections every 6 months thereafter. Boosters were introduced during the trial, thus some patients did not receive all 4 boosters. Injection sight reactions were measured.
Results: Safety data was analyzed to assess local and systemic toxicity of each treatment arm. Most subjects completed the planned PIS, 81 (91.0%) GP2+GM-CSF and 86 (94.5%) GM-CSF only. In addition, 77 GP2+GM-CSF and 80 GM-CSF only subjects received all 4 booster injections. The most common local toxicities were erythema, induration and pruritis and they occurred with similar frequency in the two treatment arms. Local reactions were reported by almost all subjects over the course of vaccinations. Occurring in a smaller percentage of subjects, the most common systemic toxicities were fatigue, headache, and myalgia/arthralgia, again with similar incidence by treatment group. The majority of all events reported were of Grade 1 mild severity (GP2+GM-CSF 92.5%, GM-CSF only 90.6%). Only 5 events in 4 subjects were considered Grade 3: induration and maculopapular rash/pruritis, in two GP2+GM-CSF subjects and chest pain and hypersensitivity reaction in two GM-CSF only subjects. The incidence of local reactions minimally increased with subsequent vaccinations; however, the types of events remain unchanged. No serious adverse events were reported over the full 5 year treatment and follow-up periods.
Conclusions: The study confirms the finding from the Phase I trial evaluating GP2+GM-CSF that the vaccine is safe and well-tolerated. The majority of patients experienced only mild local and systemic toxicities. Importantly, toxicities in the GP2+GM-CSF group were comparable to those seen in the GM-CSF only group, suggesting the toxicities are attributable to GM-CSF.
About the 2021 ASCO Annual Meeting
Founded in 1964, ASCO is the world's leading professional organization for physicians and oncology professionals caring for people with cancer. ASCO offers premier scientific events for oncology professionals, patient advocates, industry representatives, and major media outlets worldwide. The ASCO Annual Meeting program features poster presentations, poster discussion sessions, clinical science symposia, and dynamic education sessions about recent advancements in cancer research, treatment, and patient care. For more information, please visit the conference website at: https://conferences.asco.org/am/attend.
About Breast Cancer and HER2/neu Positivity
One in eight U.S. women will develop invasive breast cancer over her lifetime, with approximately 266,000 new breast cancer patients and 3.1 million breast cancer survivors in 2018. HER2/neu (human epidermal growth factor receptor 2) protein is a cell surface receptor protein that is expressed in a variety of common cancers, including in 75% of breast cancers at low (1+), intermediate (2+), and high (3+ or over-expressor) levels.
About Greenwich LifeSciences, Inc.
Greenwich LifeSciences is a clinical-stage biopharmaceutical company focused on the development of GP2, an immunotherapy to prevent breast cancer recurrences in patients who have previously undergone surgery. GP2 is a 9 amino acid transmembrane peptide of the HER2/neu protein. In a randomized, single-blinded, placebo-controlled, multi-center (16 sites led by MD Anderson Cancer Center) Phase IIb clinical trial, no recurrences were observed in the HER2/neu 3+ adjuvant setting after median 5 years of follow-up, if the patient received the 6 primary intradermal injections over the first 6 months (p = 0.0338). Of the 138 patients that have been treated with GP2 to date over 4 clinical trials, GP2 treatment was well tolerated and no serious adverse events were observed related to GP2 immunotherapy. Greenwich LifeSciences is planning to commence a Phase III clinical trial using a similar treatment regime as the Phase IIb clinical trial. For more information on Greenwich LifeSciences, please visit the Company’s website at www.greenwichlifesciences.com and follow the Company's Twitter at https://twitter.com/GreenwichLS.
Forward-Looking Statement Disclaimer
Statements in this press release contain “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” "will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on Greenwich LifeSciences Inc.’s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including statements regarding the intended use of net proceeds from the public offering; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled “Risk Factors” in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Greenwich LifeSciences, Inc. undertakes no duty to update such information except as required under applicable law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210607005374/en/
Greenwich LifeSciences Publishes Additional Positive Safety Data from GP2 Phase IIb Trial at ASCO 2021, Confirming that GP2 Treatment to Prevent Metastatic Breast Cancer Recurrence is Well Tolerated
Contacts
Company Contact
Snehal Patel
Investor Relations
(832) 819-3232
info@greenwichlifesciences.com
Investor & Public Relations Contact for Greenwich LifeSciences
Dave Gentry
RedChip Companies Inc.
Office: 1-800-RED CHIP (733 2447)
Cell: (407) 491-4498
dave@redchip.com
More News
View More




Recent Quotes
View MoreQuotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.



