Financial News
PureTech Acquires Remaining Interest in Founded Entity Alivio Therapeutics
Oral IL-22 and other preclinical therapeutic candidates as well as the underlying technology platform to be integrated in the Company’s Wholly Owned Pipeline
PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced the acquisition of the remaining 22 percent of shares outstanding in its Founded Entity, Alivio Therapeutics (“Alivio”). Alivio’s therapeutic candidates, in development for inflammatory disorders including inflammatory bowel disease (IBD), will be integrated into the Company’s Wholly Owned Pipeline.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210616005153/en/
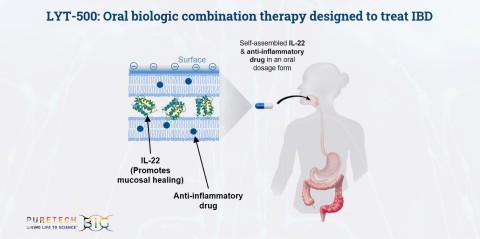
PureTech announced the acquisition of the remaining interest in its Founded Entity, Alivio Therapeutics. Alivio’s therapeutic candidates, in development for inflammatory disorders including inflammatory bowel disease (IBD), will be integrated into the Company’s Wholly Owned Pipeline, including the addition of LYT-500, an orally administered therapeutic candidate in development for the treatment of IBD. (Graphic: Business Wire)
The acquisition aligns with PureTech’s strategy to advance a Wholly Owned Pipeline designed to harness key immunological, fibrotic and lymphatic system mechanisms to treat serious diseases with significant unmet needs. The programs and the underlying AlivioTM technology platform are expected to be funded by PureTech as well as through partnerships and grants. The integration of this program is in line with the budget for PureTech, which extends into the first quarter of 2025, as previously guided.
“PureTech founded Alivio alongside leading scientists Jeffrey Karp, Ph.D., Professor of Medicine at Brigham and Women’s Hospital and Robert Langer, Sc.D., David H. Koch Institute Professor at MIT to pioneer a novel strategy to more effectively treat inflammatory disorders through highly targeted immunomodulation at the site of disease. This promising approach fits well within our Wholly Owned Pipeline and we will be able to leverage our strength in immunology and related technologies as we progress therapeutic candidates to potentially treat inflammatory diseases,” said Daphne Zohar, Founder and Chief Executive Officer of PureTech. “We’re pleased to add the Alivio programs to our pipeline and proud to advance a platform that we hope will bring new therapeutic options to millions of people with chronic and life-limiting autoimmune and inflammatory diseases.”
The Wholly Owned Pipeline will include the addition of LYT-500, an orally-administered therapeutic candidate in development for the treatment of IBD. Utilizing the Alivio technology platform, LYT-500 consists of two active agents intended to selectively act at the inflamed tissues while reducing their impact on the normal tissue. LYT-500 contains a unique combination of IL-22 and an anti-inflammatory drug, which is designed to address the two key underlying causes of IBD pathogenesis and progression, namely mucosal barrier disruption and inflammation.
Existing biologic therapies indicated for IBD must be provided through multiple injections over time and are associated with several limitations including loss of efficacy over time and increased risk for opportunistic infections. Using the Alivio technology platform, a biologic agent and small molecule drug can be combined into a single oral dosage form that offers the potential for enhancing the treatment of inflamed tissues to maximize efficacy, while reducing systemic exposure to minimize toxicity. Unlike other therapies in development for IBD, LYT-500 has the potential to provide an oral drug therapy that targets multiple mechanisms of disease pathogenesis, while reducing the potential for systemic side effects.
The integration also includes the addition of therapeutic candidate, LYT-503/IMB-150, to the Company’s pipeline, which is being developed in collaboration with Imbrium Therapeutics as a potential non-opioid treatment for interstitial cystitis or bladder pain syndrome (IC/BPS). An IND filing for LYT-503/IMB-150 is expected in 2021. PureTech will also continue to evaluate existing and additional anti-inflammatory programs leveraging the Alivio platform technology.
PureTech’s Wholly Owned Pipeline also includes three clinical-stage programs: LYT-100, a selectively deuterated form of pirfenidone that has demonstrated anti‑inflammatory and anti-fibrotic activity and is being advanced for idiopathic pulmonary fibrosis and potentially other PF-ILDs, where registration-enabling studies are being planned and is currently being evaluated in a Phase 2 trial to treat respiratory complications and related sequelae of Long COVID as well as a Phase 2a proof-of-concept study in patients with breast cancer-related, upper limb secondary lymphedema; and LYT-200, a monoclonal antibody targeting immuno-suppressive galactin-9, which is in a Phase 1 trial for metastatic solid tumors. PureTech’s Wholly Owned Pipeline also includes LYT-300 an oral version of allopregnanolone (a natural neurosteroid), developed using PureTech’s proprietary Glyph platform, and several other discovery platforms leveraging the company’s expertise in lymphatic targeting.
The consideration for the acquisition of the minority interests in Alivio consist of a closing cash payment and potential future cash payments upon successful achievement of certain milestones. The transaction is a small transaction for the purposes of Annex 1 of Listing Rule 11 and smaller than a class 2 transaction for the purposes of Listing Rule 10.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including inflammatory, fibrotic and immunological conditions, intractable cancers, lymphatic and gastrointestinal diseases and neurological and neuropsychological disorders, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 26 therapeutics and therapeutic candidates, including two that have received FDA clearance and European marketing authorization, as of the date of PureTech’s most recently filed Annual Report on Form 20-F. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company's unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, our expectations regarding the potential therapeutic benefits of our therapeutic candidates, our expectations regarding the acquired minority interest in Alivio Therapeutics, the acquired therapeutic candidates including the potential benefits therefrom, and those risks and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210616005153/en/
Contacts
Investors
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
U.S. media
Stephanie Simon
+1 617 581 9333
stephanie@tenbridgecommunications.com
More News
View More



Recent Quotes
View MoreQuotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.



