Financial News
Fresenius Kabi Introduces New Generic for the Treatment of Non-Squamous Non-Small Cell Lung Cancer
Expanding access to oncology medicines
Fresenius Kabi announced today the immediate availability in the U.S. of PEMEtrexed for Injection, USP, a new generic equivalent to Alimta®. Fresenius Kabi produces its PEMEtrexed for Injection, USP in the United States, where the company offers the most comprehensive injectable oncology portfolio of any pharmaceutical manufacturer.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220531005701/en/
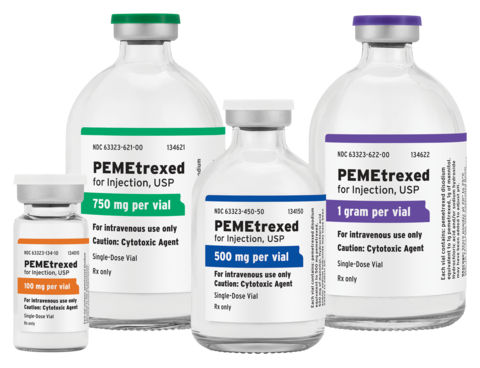
Fresenius Kabi PEMEtrexed for Injection, USP (Photo: Business Wire)
Fresenius Kabi PEMEtrexed for Injection, USP is an affordable treatment option for adult patients with non-squamous non-small cell lung cancer and malignant pleural mesothelioma.
Available in four presentations for intravenous (IV) use, Fresenius Kabi now has the broadest portfolio of PEMEtrexed for Injection, USP products available from any manufacturer in the U.S., including two new strengths designed to help streamline pharmacy operations and reduce drug waste:
- PEMEtrexed for Injection, USP 100 mg per single-dose vial
- PEMEtrexed for Injection, USP 500 mg per single-dose vial
- New strength: PEMEtrexed for Injection, USP 750 mg per single-dose vial
- New strength: PEMEtrexed for Injection, USP 1 gram per single-dose vial
“We are pleased to introduce another generic chemotherapy medication to help make cancer treatments more accessible and affordable for patients,” said John Ducker, president and CEO of Fresenius Kabi USA. “And we’re proudly producing our PEMEtrexed for Injection here in the U.S.”
Fresenius Kabi offers more than 30 different oncology drugs in the U.S., and nearly 90 percent are formulated, filled and finished in the U.S. PEMEtrexed for Injection, USP is the newest example of the company’s commitment to investing “More in America.” This effort is focused on providing more supply, more science, more support and more care to its customers and the patients they serve in the U.S. Fresenius Kabi has invested nearly $1 billion to modernize and expand advanced U.S. pharmaceutical production and distribution facilities.
PEMEtrexed for Injection, USP, along with other oncology medicines, is part of the company’s KabiConnect program, a recent expansion of its KabiCare patient support program that offers copay assistance to eligible U.S. patients. The program can lower out-of-pocket costs to as little as $0 per month for eligible patients. To determine eligibility, patients should speak to their physician. Enrollment is a simple online process. Details can be found on the KabiCare website at kabicare.us.
Important Safety Information
INDICATIONS AND USAGE
Pemetrexed for Injection is a folate analog metabolic inhibitor indicated:
- in combination with pembrolizumab and platinum chemotherapy, for the initial treatment of patients with metastatic non-squamous NSCLC, with no EGFR or ALK genomic tumor aberrations.
- in combination with cisplatin for the initial treatment of patients with locally advanced or metastatic, non-squamous, non-small cell lung cancer (NSCLC).
- as a single agent for the maintenance treatment of patients with locally advanced or metastatic, non-squamous NSCLC whose disease has not progressed after four cycles of platinum-based first-line chemotherapy.
- as a single agent for the treatment of patients with recurrent, metastatic non-squamous, NSCLC after prior chemotherapy. Limitations of Use: Pemetrexed for Injection is not indicated for the treatment of patients with squamous cell, non-small cell lung cancer.
- initial treatment, in combination with cisplatin, of patients with malignant pleural mesothelioma whose disease is unresectable or who are otherwise not candidates for curative surgery.
IMPORTANT SAFETY INFORMATION
Pemetrexed for Injection is contraindicated in patients with a history of severe hypersensitivity reaction to pemetrexed.
Myelosuppression: Can cause severe bone marrow suppression resulting in cytopenia and an increased risk of infection. Do not administer pemetrexed for injection when the absolute neutrophil count is less than 1500 cells/mm3 and platelets are less than 100,000 cells/mm3. Initiate supplementation with oral folic acid and intramuscular vitamin B12 to reduce the severity of hematologic and gastrointestinal toxicity of pemetrexed for injection.
Renal Failure: Can cause severe, and sometimes fatal, renal failure. Do not administer when creatinine clearance is less than 45 mL/min.
Bullous and Exfoliative Skin Toxicity: Permanently discontinue for severe and life-threatening bullous, blistering or exfoliating skin toxicity.
Interstitial Pneumonitis: Withhold for acute onset of new or progressive unexplained pulmonary symptoms. Permanently discontinue if pneumonitis is confirmed.
Radiation Recall: Can occur in patients who received radiation weeks to years previously; permanently discontinue for signs of radiation recall.
Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and to use effective contraception.
The most common adverse reactions (incidence ≥ 20%) of pemetrexed for injection, when administered as a single agent are fatigue, nausea, and anorexia.
The most common adverse reactions (incidence ≥ 20%) of pemetrexed for injection when administered with cisplatin are vomiting, neutropenia, anemia, stomatitis/pharyngitis, thrombocytopenia, and constipation.
The most common adverse reactions (incidence ≥ 20%) of pemetrexed for injection when administered in combination with pembrolizumab and platinum chemotherapy are fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, dyspnea, and pyrexia.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Ibuprofen increased risk of pemetrexed for injection toxicity in patients with mild to moderate renal impairment. Modify the ibuprofen dosage as recommended for patients with a creatinine clearance between 45 mL/min and 79 mL/min.
Lactation: Advise not to breastfeed.
This Important Safety Information does not include all the information needed to use Pemetrexed for Injection, USP safely and effectively. Please see full prescribing information for Pemetrexed for Injection, USP at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion, and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at www.fresenius-kabi.com/us/join-us and follow us on LinkedIn.
Alimta® is a registered trademark of Eli Lilly and Company
View source version on businesswire.com: https://www.businesswire.com/news/home/20220531005701/en/
#FreseniusKabi announces U.S. availability of PEMEtrexed for Injection, USP.
Contacts
Media contact
Joanie Clougherty (614) 717-5741
joan.clougherty@fresenius-kabi.com
Stock quotes supplied by Barchart
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms and Conditions.



