Financial News
Canary Cure Therapeutics Unveils Breakthrough Human Cell Data for Next-Generation Obesity Therapy, CCT-217, Demonstrating Muscle-Building Fat Loss and Paving Path to Clinic
Dual siRNA Therapeutic Confirms Its Robust In Vivo Results, Strongly De-Risking the Clinical Path and Underscoring CCT-217's Potential as a Transformative, Patient-Friendly Therapy for Obesity and Metabolic Disorders
BOSTON, MASSACHUSETTS / ACCESS Newswire / July 10, 2025 / Canary Cure Therapeutics (Canary), a pioneering biopharmaceutical company, today announced groundbreaking human cell data for its lead therapeutic candidate, CCT-217. The findings confirm a unique mechanism that promotes rapid, selective fat loss while preserving and building muscle. This strong human and preclinical evidence de-risks the clinical path for a transformative, twice-yearly treatment for obesity, with Phase 1 trials targeted for 2026.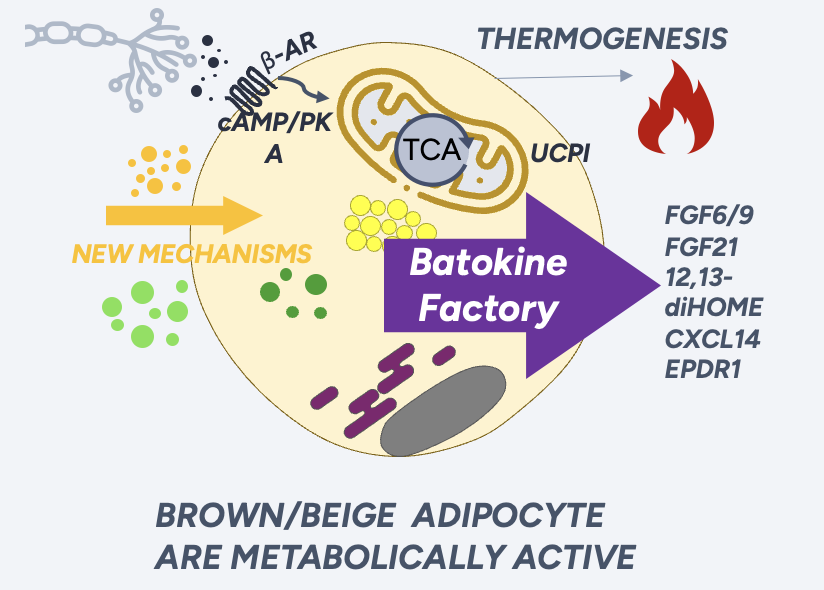
The global obesity market is projected to exceed $200 billion by 2031, yet current treatments primarily rely on appetite suppression, often leading to significant loss of healthy muscle. CCT-217 represents a paradigm shift by targeting the underlying metabolic causes of obesity.
"While weight loss can be achieved by decreasing calorie intake, we believe increasing the body's ability to burn fat - especially harmful visceral fat - is a fundamentally healthier approach," said Raj Reddy, CEO of Canary. "Our goal is to improve body composition and overall metabolic function."
Developed from Canary's proprietary dual siRNA platform, CCT-217 is engineered to increase energy expenditure by converting energy-storing white fat into metabolically active, energy-burning beige fat.
Highlights From Recent Data:
Human Cell Data Confirms Mechanism: Recent studies on differentiated human white fat cells treated with CCT-217 showed a rapid and significant increase in key thermogenic gene markers within just 72 hours. This powerful result in human cells validates the therapy's core mechanism and potential efficacy, providing a clear path toward clinical trials.
-
Dramatic, Fat-Selective Weight Loss in Preclinical Models: In diet-induced obese (DIO) animal models, CCT-217 demonstrated remarkable results in only 20 days:
26% reduction in total body weight
60% reduction in harmful visceral fat
25% increase in lean muscle mass
Comprehensive Metabolic Health Improvements: Beyond weight loss, CCT-217 treatment led to a complete reversal of liver steatosis (fatty liver), a 20% reduction in cholesterol, and enhanced insulin sensitivity, underscoring its potential to address a wide range of obesity-related co-morbidities.
CCT-217 offers patient-friendly, twice-yearly subcutaneous injections, using a proven Lipid Nanoparticle (LNP) platform to target siRNA delivery to adipose tissue - an endocrine organ central to the development of metabolic diseases - while minimizing off-target effects. Canary's second asset, CCT-007, targets genes over-expressed in post-menopausal women for healthy weight loss with fewer side effects.
"The strong correlation between our new human cell data and the dramatic in vivo animal model outcomes provides critical de-risking evidence," said Dr. Reddy. "We are now focused on advancing CCT-217 into Phase 1 clinical trials in 2026 to bring this next-generation therapy to patients. We are fortunate to have exceptional advisors overseeing the clinical development including Dr. Michael Lincoff, Principal Investigator of the groundbreaking SELECT Trial."
Canary is currently raising a Series A round of $25 million to advance CCT-217 into Phase 1 trials, expected to start in 2026.
About Canary Cure Therapeutics
Canary Cure Therapeutics is a late-stage preclinical biopharmaceutical company pioneering next-generation RNA therapeutics for obesity and metabolic disease. Its proprietary dual-siRNA platform is engineered to induce class-leading thermogenesis, offering a differentiated, muscle-sparing approach to weight loss and metabolic health.
Contact Information
Anna Wang
Chief Strategy Officer
anna@canarycure.com
+41 79 204 2875
SOURCE: Canary Cure Therapeutics
View the original press release on ACCESS Newswire
More News
View More





Quotes delayed at least 20 minutes.
By accessing this page, you agree to the following
Privacy Policy and Terms Of Service.



